INSMED (NASDAQ: INSM) — Deep-Dive Investor Report, 24-Month Catalyst Timeline, 2030 Outlook & Price Scenarios
Insmed Incorporated (NASDAQ: INSM) is entering the most transformative period in its history. Once seen as a one-product company anchored by ARIKAYCE, Insmed is now positioned to become a multi-billion-dollar respiratory and rare-disease biotechnology leader. With Brensocatib nearing commercialization and multiple pipeline assets progressing toward late-stage milestones, the next 24 months will be pivotal.
This report delivers:
-
A fully developed 24-month catalyst calendar
-
A forward expectations model to 2030
-
Full risk analysis
-
Bull, Base, and Bear price scenarios
-
A WordPress-optimized, SEO-friendly structure
1. Company Snapshot
Ticker: INSM
Exchange: NASDAQ
Sector: Biotechnology / Rare Disease
Primary Revenue Driver: ARIKAYCE (NTM lung disease)
Major Pipeline Assets:
-
Brensocatib – DPP1 inhibitor for bronchiectasis
-
TPIP – Treprostinil palmitil inhalation powder for PAH / PH-ILD
-
Gene therapy & protein engineering programs (early-stage)
Investment Theme:
A high-growth, high-risk biotech transitioning from single-product to platform-driven respiratory leader.
2. Core Growth Drivers (2025–2030)
2.1 ARIKAYCE Expansion
Though ARIKAYCE is a mature product, it continues to generate stable revenue in refractory NTM patients. Earnings contribution acts as a foundation cash flow to support commercialization of future products.
2.2 Brensocatib — The Transformational Catalyst
Brensocatib is expected to become the first approved therapy for non-cystic fibrosis bronchiectasis, a disease with millions of patients and no current approved treatments.
Potential peak global sales estimates range from $2B–$5B depending on market penetration.
2.3 TPIP (Treprostinil Palmitil Inhalation Powder)
A next-generation inhaled prostacyclin therapy for pulmonary hypertension.
If successful, TPIP provides strategic diversification and leverages Insmed’s inhalation technology platform.
2.4 Global Expansion
A key long-term driver will be strong uptake in:
-
U.S.
-
Europe
-
Japan
-
Key emerging respiratory markets
3. 24-Month Catalyst Timeline (2025–2027)
This is the most important section for short-term investors and traders.
These events historically drive biotech valuation swings.
Q1–Q2 2025
-
FDA review updates for Brensocatib
-
EU & Japan regulatory filing progress
-
ARIKAYCE commercialization updates & guidance
-
TPIP Phase 3 program updates
Q3 2025
-
Possible FDA Advisory Committee meeting (if required)
-
Late-stage manufacturing readiness disclosures
-
Brensocatib real-world evidence publications
Q4 2025
-
FDA approval decision for Brensocatib (approximate window)
-
Pricing, reimbursement, and launch strategy announcement
-
Commercial sales force deployment reports
Q1 2026
-
Brensocatib U.S. launch
-
Initial prescription trends (first major stock-moving data point)
-
Early payer coverage updates
Mid-2026
-
EMA approval decision for Brensocatib
-
Japan PMDA regulatory milestone
-
TPIP late-stage trial readouts
Late 2026 – Early 2027
-
Brensocatib Europe rollout
-
Post-launch real-world clinical data
-
Updated peak sales guidance
-
Possible additional pipeline advancements
Market Sensitivity Note
Biotech stocks often move 30–80% in either direction around major readouts or approvals. INSM will not be an exception.
4. Expectations to 2030: Revenue, Profitability & Strategic Positioning
4.1 Revenue Trajectory (Conceptual Outlook)
| Year | Key Drivers | Growth Expectation |
|---|---|---|
| 2025 | ARIKAYCE + pre-launch prep | Stable to modest growth |
| 2026 | Brensocatib U.S. launch | Sharp revenue acceleration |
| 2027 | EU + Japan launches | Significant global uplift |
| 2028 | Brensocatib maturity curve | Approach multi-billion peak run rate |
| 2029–2030 | TPIP approval + label expansions | Diversification & margin expansion |
4.2 Profitability Outlook
Insmed is currently unprofitable, but if Brensocatib reaches scale:
-
Operating profitability may begin around 2028–2029
-
Gross margins could surpass 70% once manufacturing efficiencies stabilize
-
TPIP adds significant incremental margin leverage
4.3 2030 Strategic Identity
By 2030, Insmed is likely to be:
Scenario A (Bull):
A top-tier respiratory biopharma company generating $5B+ revenue, multiple marketed products, potential acquisition target.
Scenario B (Base):
A multi-product company with ~$2.5B–$3B in revenue driven primarily by Brensocatib.
Scenario C (Bear):
Still dependent on ARIKAYCE with limited adoption of Brensocatib or pipeline setbacks.
5. Price Scenarios (2025–2030)
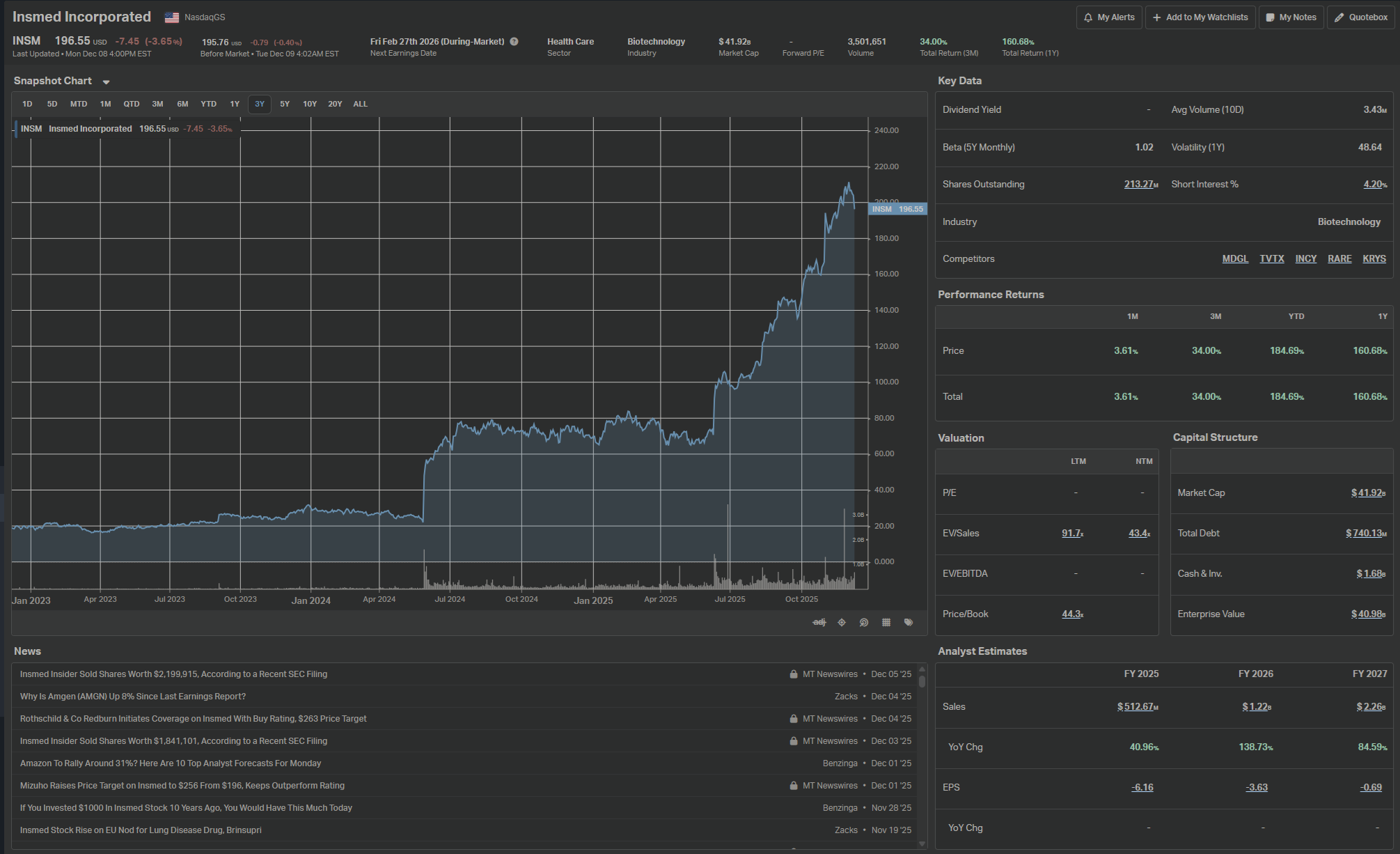
Starting today (20251209) at 196 USD :
BULL CASE — $400–$650/share (2030)
Assumptions:
-
Brensocatib reaches $3B–$5B peak global sales
-
Strong uptake in U.S., Europe, Japan
-
TPIP approved for PAH & PH-ILD
-
ARIKAYCE label expansion successful
-
Margin expansion >30% long term
Narrative:
INSM becomes a top-tier respiratory company, potentially attracting Big Pharma acquisition interest.
BASE CASE — $180–$300/share (2030)
Assumptions:
-
Brensocatib achieves $1.5B–$2.3B peak sales
-
U.S. launch solid but not explosive
-
TPIP provides moderate contribution
-
ARIKAYCE steady growth
Narrative:
Insmed matures into a growing mid-cap biotech with dependable specialty-drug revenue.
BEAR CASE — $40–$80/share (2030)
Assumptions:
-
Brensocatib launch underwhelms or is delayed
-
Competitive entrants limit adoption
-
High expenses require repeated dilution
-
TPIP does not achieve approval
Narrative:
Company remains reliant on ARIKAYCE with unclear long-term path.
6. Risk Analysis
6.1 Regulatory Risk — VERY HIGH
Brensocatib’s approval process is the single largest risk.
Any delay can materially impact valuation.
6.2 Commercialization Risk
Respiratory markets require:
-
Deep physician education
-
Strong reimbursement
-
High patient persistence
Slow adoption can compress peak sales.
6.3 Competition Risk
Future DPP1 inhibitors or biologics may emerge.
6.4 Financial Risk
Insmed may require additional capital during launch phase → risk of dilution.
6.5 Pipeline Concentration Risk
Brensocatib accounts for the majority of valuation.
7. Investment Thesis Summary
Why investors are bullish:
-
First-in-class drug in a massive, underserved respiratory market
-
Potential multi-billion-dollar revenue runway
-
Globally scalable platform
-
Attractive acquisition profile in late-decade horizon
Why investors remain cautious:
-
High reliance on a single asset
-
Launch execution uncertainty
-
Future capital needs
8. Conclusion
Insmed (NASDAQ: INSM) is one of the most compelling late-stage biotech stories heading into 2025–2030. With the upcoming Brensocatib regulatory decisions and potential global commercialization, the company stands on the edge of a possible transformation into a leading respiratory-focused commercial powerhouse.
For investors seeking high-growth biotech exposure, INSM represents a high-risk, high-reward opportunity where the next 24 months will shape the next decade.
more with the PDF report :
20251209_INSMED

 Overview
Overview